Asheville, North Carolina, September 12, 2025
News Summary
Mission Hospital, in partnership with the HCA Healthcare Research Institute, has successfully completed a pivotal phase of a clinical trial aimed at lowering stroke risks in patients undergoing transcatheter aortic valve replacement (TAVR). The trial utilizes the innovative EmStop™ device, designed to prevent debris from entering the bloodstream during procedures. With ten successful cases performed at Mission Hospital and five at TriStar Centennial Medical Center, this technology could redefine standards in cardiac care, potentially benefiting patients worldwide.
Asheville, North Carolina – Mission Hospital, in collaboration with the HCA Healthcare Research Institute, has completed an essential phase of a pioneering national clinical trial aimed at reducing stroke risks in patients undergoing cardiac surgery. This trial marks a significant advancement in medical technology, especially concerning transcatheter aortic valve replacement (TAVR), a procedure that offers a less invasive alternative to traditional open-heart surgery.
The main focus of this clinical trial is to mitigate the risk of stroke, which is a notable concern for patients undergoing TAVR. Historically, between 2% to 3% of patients have experienced a stroke within 72 hours post-operation due to dislodged debris during the procedure. With this new approach, researchers hope to greatly reduce this statistic through the use of a novel stroke protection device.
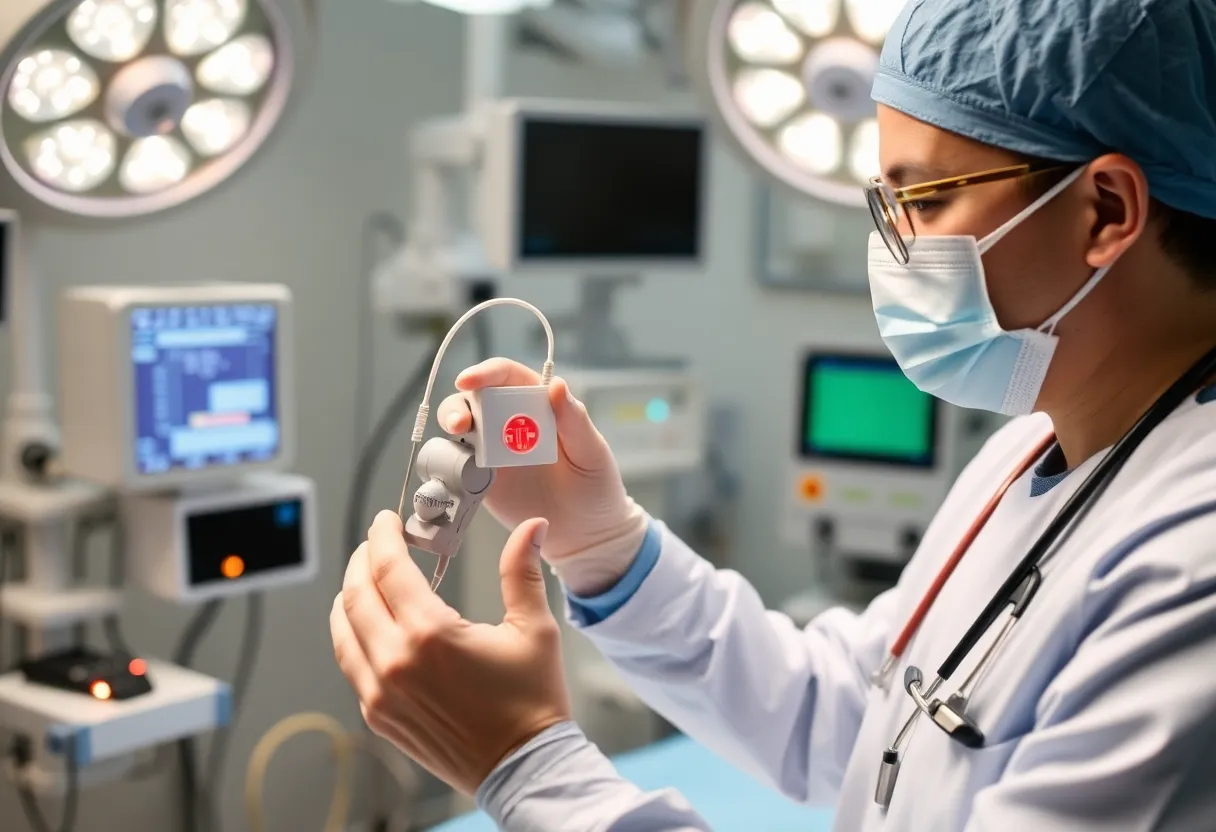
The device, known as EmStop™, is the first fully integrated embolic protection system specifically designed for TAVR procedures. Its purpose is to prevent potentially harmful debris from entering the bloodstream during the valve replacement process. Developed by a local cardiac surgeon, EmStop™ has already shown promise through successful testing at Mission Hospital and the HCA Healthcare affiliate, TriStar Centennial Medical Center.
A total of ten successful cases with the EmStop™ device were performed at Mission Hospital, along with five additional cases at the TriStar facility. The trial aims to expand to select hospitals nationwide, should the ongoing results continue to be favorable. Dr. Michael Chenier, the Principal Investigator, views this trial as a crucial leap forward in cardiac care that could set new global standards for medical practices in the field.
Mission Hospital, part of a progressive learning health system, employs real-world data and clinical studies to usher in scientific innovations. It currently has over 35 active clinical trials under the HCA Healthcare Research Institute umbrella, of which 25 focus specifically on various facets of cardiac care.
If the outcomes of the ongoing trial remain favorable, the EmStop™ device is positioned to become the gold standard in valve replacement procedures, significantly altering the scope of treatment options available to cardiac surgery patients worldwide.
Supporting Details
The success of the EmStop™ device is not just a victory for Mission Hospital and its patients, but a potential game changer in the realm of cardiac surgery. Traditional approaches to TAVR have carried a risk of complications, including strokes, which can dramatically affect recovery and overall health outcomes. With the EmStop™ device, there is hope that these risks can be significantly minimized.
The clinical trial is part of a broader initiative by HCA Healthcare to leverage the latest research and technology to improve patient care through innovative practices. This cooperative research signifies a commitment to enhancing patient outcomes across various medical procedures, particularly those involving high-risk cardiovascular interventions.
Background Context
TAVR procedures have seen a steady rise in popularity due to their less invasive nature compared to traditional open-heart surgery. However, concerns about stroke risk remain a significant barrier to wider adoption. The development of devices like EmStop™ is a response to this challenge, using innovative technology to create safer surgical environments. As Mission Hospital progresses with its clinical trials, the implications for cardiac surgery could be profound, potentially setting new standards and practices for hospitals across the globe.
FAQ Section
What is the primary purpose of the clinical trial conducted at Mission Hospital?
The clinical trial aims to reduce the risk of stroke in patients undergoing transcatheter aortic valve replacement (TAVR) procedures.
What is the EmStop™ device?
EmStop™ is the first fully integrated embolic protection system designed specifically for TAVR procedures, aimed at preventing harmful debris from entering the bloodstream.
How many cases have been performed using the EmStop™ device?
A total of ten successful cases were completed at Mission Hospital, with an additional five cases performed at TriStar Centennial Medical Center.
What does the success of the clinical trial mean for future cardiac surgeries?
If the trial outcomes remain favorable, the EmStop™ device may become the gold standard in valve replacement procedures, improving safety and outcomes for patients worldwide.
How many clinical trials are ongoing at Mission Hospital?
Mission Hospital currently has over 35 active clinical trials, with 25 dedicated to various aspects of cardiac care.
Key Features of the EmStop™ Device
| Feature | Description |
|---|---|
| Innovative Design | First fully integrated embolic protection system for TAVR. |
| Stroke Prevention | Aims to reduce stroke risk associated with dislodged debris during procedures. |
| Clinical Success | Ten successful cases at Mission Hospital and five at TriStar Centennial Medical Center. |
| Future Standard | Potential to become the gold standard in valve replacement procedures. |
| Part of a Larger Initiative | Located within a learning health system focused on driving scientific breakthroughs. |
Deeper Dive: News & Info About This Topic
HERE Resources
Additional Resources
- 828 News Now: Tomorrow’s Medical Breakthroughs
- WLOS: Western NC Hospital Pioneers Stroke Prevention Device
- US News: Mission Hospital Overview
- Google Search: Transcatheter Aortic Valve Replacement
- Wikipedia: General Information

Author: STAFF HERE ASHEVILLE WRITER
The ASHEVILLE STAFF WRITER represents the experienced team at HEREAsheville.com, your go-to source for actionable local news and information in Asheville, Buncombe County, and beyond. Specializing in "news you can use," we cover essential topics like product reviews for personal and business needs, local business directories, politics, real estate trends, neighborhood insights, and state news affecting the area—with deep expertise drawn from years of dedicated reporting and strong community input, including local press releases and business updates. We deliver top reporting on high-value events such as the Asheville Bread Festival, LEAF Festival, and mountain sports tournaments at Biltmore Estate. Our coverage extends to key organizations like the Asheville Area Chamber of Commerce and Explore Asheville Convention & Visitors Bureau, plus leading businesses in hospitality and brewing that power the local economy such as the Biltmore Estate and Sierra Nevada Brewing Company. As part of the broader HERE network, including HERECharlotte.com, HEREGreensboro.com, HERERaleigh.com, and HEREOBX.com, we provide comprehensive, credible insights into North Carolina's dynamic landscape.